Participate in an outpatient vaccine study
What to expect?
Sign up for a study that suits you
Find a study that suits you, for which you match the target group and sign up.
We will contact you to discuss your registration and we will invite you for a screening visit.
This screening invitation is free of any obligation.


Screening visit
During the screening visit you will be asked to sign the informed consent form (ICF) after you have been able to ask all your questions, and everything is clear.
After signing the ICF, we will check your health and whether you meet all the study specific conditions for participation. We will ask questions about your medical history and medication use, take samples (blood, urine and or nasal swabs), perform a full physical assessment and measure your vital signs, weight and height.
Participating in the study
If you fulfill the criteria and you are still willing to participate, you can be enrolled in the study.
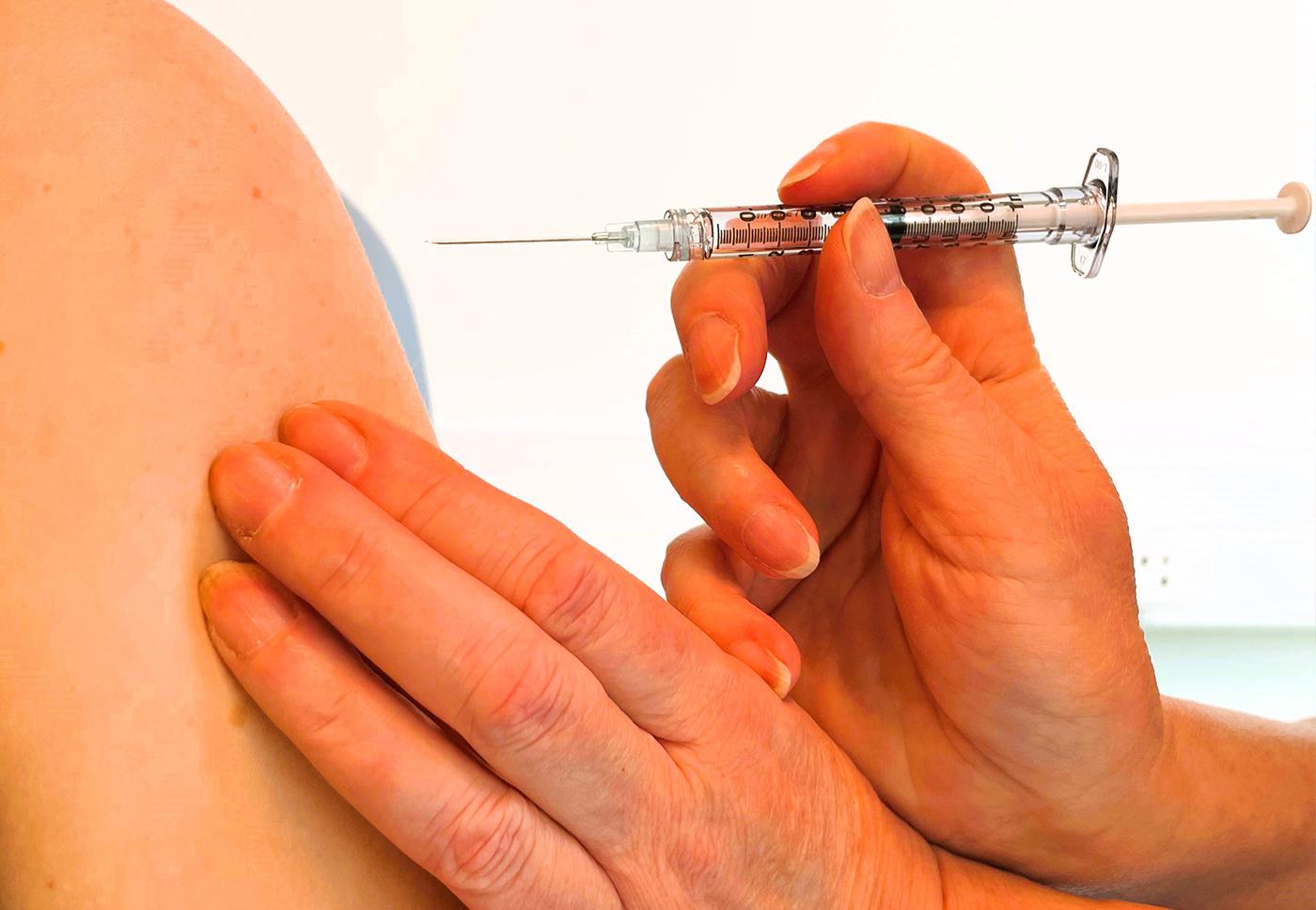
At the next visit, which we call the baseline visit you will receive a vaccination in the presence of a physician or nurse.
After vaccination, you will be asked to come back for several visits at pre-planned times to monitor your health. We will perform physical examinations and collect blood and urine samples to assess the safety and effect of the vaccine. For some studies, you will also be asked to keep a diary to document your symptoms .
These outpatient visits generally take between 1 to 4 hours after which you can go home.



Final follow-up visit
The study will end by performing the final follow-up visit where we will perform assessments like the ones performed at the screening visit to make sure that you are completely healthy.
Fair Compensation (tax free in Belgium)
Provided as a compensation for your time, travel and involvement.
Up to €150 per ambulatory visit, with the final amount depending on the study design.
Every 3 months, based on the actual visits performed during that period, the corresponding amount is transferred to your bank account.